Workflows
Download: Preformulation and formulation support | 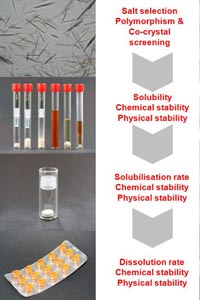 |
Analytics
Downloads: |  |
Process Analytics (PAT)
|  |
Workflows
Download: Preformulation and formulation support |  |
Analytics
Downloads: |  |
Process Analytics (PAT)
|  |